![]()
Industrial Partners:
| 8220847 |
Developed by Baumeister Mediasoft Engineering
|
HomeLogosNewsSearchSitemapContact |
EFHSS Conference 2002 - Lectures
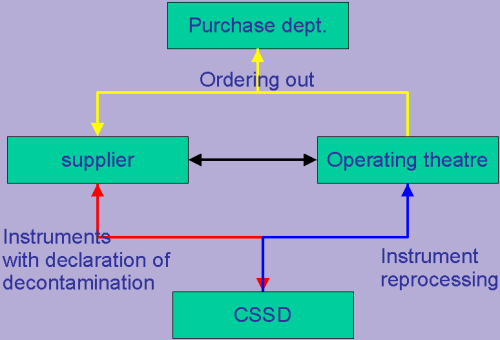
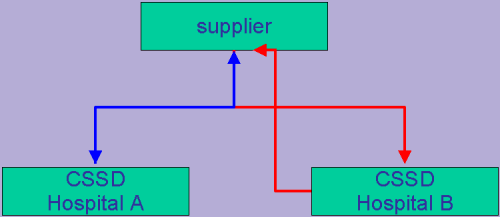
Loan Instruments
The following slide show was presented by René Vis (CSC - The Netherlands) at the EFHSS Conference 2002 in Dublin.
|
|
|
|
|
|
|
|
|
|
|
|
|
|