The Sterile Supply Cycle: Packaging
Author: Jan Huys
| TOP | CONTENTS | PREVIOUS | NEXT | BOTTOM |
Packaging of Sterile Products
|
|
|||||||||||||||||||
 |
|
||||||||||||||||||
| TOP | CONTENTS | PREVIOUS | NEXT | BOTTOM |
Contents
|
   |
| TOP | CONTENTS | PREVIOUS | NEXT | BOTTOM |
Why packing of sterile goods?
 Packaging is essential for maintaining sterility! |
At the end of a correct sterilization process, products inside the sterilizer chamber are sterile. The air in the room where the sterilizer is installed contains dust particles, which may carry micro-organisms. So when taking out the load from the sterilizer, it soon will be contaminated again. In addition usually sterile goods are stored for quite some time before they are used, and moreover they are transported through the hospital to the place they are to be used. It thus is obvious, that, when not protected, the goods will definitely be recontaminated by the time they are used. The goods must be put in a packaging to prevent recontamination after sterilization. At the same time the packaging should be suitable for sterilizing the goods it contains! Moreover the packaging protects is content against damage during handling and transport. |
Any products that are to be sterilized, have to be packed.
| TOP | CONTENTS | PREVIOUS | NEXT | BOTTOM |
Packaging concept for sterile products
Depending on use, storage and transportation, a sterile product should be packaged in one or more packaging layers:
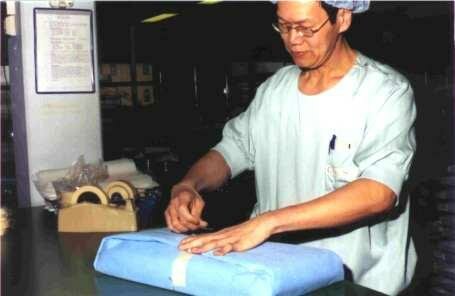
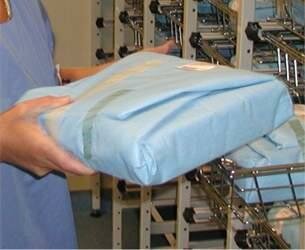 An instrument set in its primary packaging |
Primary packaging containing the productThe primary packaging prevents recontamination of the product after sterilization. It should provide an effective microbial barrier and it should allow passage of air and the sterilizing agent, e.g. steam. A primary packaging is sufficient in situations that there is no chance that dust is deposited on the pack. In a dust free storage or in case of immediate use of the materials. The primary packaging maintains sterility during storage and transport. Examples of primary packaging: 2 layers of paper, 2 layers of non-woven sheets, single or double laminated film pouch, paper bag or container with adequate filter(s). |
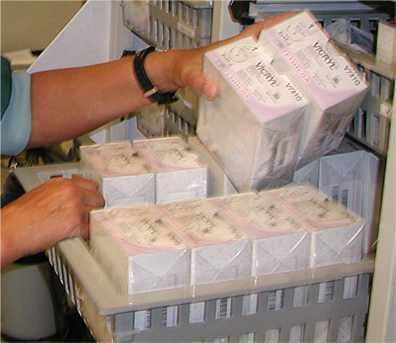 Disposable sterile items in secondary packaging |
Secondary packagingThis second layer used to facilitate proper storage and internal transport to user. It may contain one or more primary packages. E.g. an extra, plastic wrapper, bag, cardboard box or container. It may offer extra protection against dust and gives extra mechanical protection, making handling easier. Commonly used for disposable sterile supplies |
 Closed trolleys for external transport |
Transport packagingA transport package is used for external transporting of sterile goods in their primary and secondary packaging. Usually it is a strong cardboard box, cradle, closed trolley or other type of container. When the goods enter the clean area, e.g. operating theatre, the transport packaging should be removed. |
Any product to be sterile should be sterilized
at least in a primary packaging
| TOP | CONTENTS | PREVIOUS | NEXT | BOTTOM |
Validation of the packaging system
 Any combination of load, packaging and sterilization process should be validated. |
It is essential that a packaging system with its content meet the requirements in terms of sterility maintenance and protection of its contents. That is why any packaging should be validated in combination with the actual load and the sterilization process used. When testing a packaging system, also handling transport and storage conditions should be considered! (see EN 868-1) |
| TOP | CONTENTS | PREVIOUS | NEXT | BOTTOM |
Requirements for the primary packaging
When observing a packaging from the moment it is put around a product before sterilization until the moment it is opened, just before use of its content, the requirements become clear:
- Enabling sterilization. The packaging should allow air that is in the pack to be driven out and the sterilizing agent to reach all surfaces of its content.
- Compatible with the sterilization process. The packaging should be able to withstand the conditions during the sterilization process such as pressure changes, high temperature and humidity
- Maintaining Sterility. After taking the product out of the sterilizer it should remain sterile during handling, transport and storage until use.
- Strong. It should be remain intact after any intended handling and shipping
- Ensure product integrity and patient safety. It should not release any chemicals or particles or affect the product in any other way, which may affect the quality of the product or which might endanger the patient on which the product will be used.
- Indicator. The packaging should bear a clearly visible marking indicating whether or not the product is sterilized.
- Facilitate aseptic opening and presentation. When opening a packed sterile product, the chance for recontamination of the product should be kept as low as possible. When taking the product from the packaging, recontamination should be prevented. In other words: the packaging should facilitate aseptic opening and presentation. This implies:
-
- simple opening
- when taking out sterile objects form the packaging, it should not be possible to touch the unsterile outer side of the packaging.
- Visible that it was opened. Opening should result in a clearly visible indication that the pack was opened.
| TOP | CONTENTS | PREVIOUS | NEXT | BOTTOM |
General guidelines for packaging practice
- If in a health facility more than one sterilization methods are used, the accidental exchange of packaging materials shall be prevented. The packaging should have an indication showing clearly for which sterilization method it should be used.
- Reusable medical supplies that are to be re-sterilized (e.g. due to passing of the expiry date of a pack, or because of damaged packaging), should inspected, where necessary cleaned and repacked. Textile should be reconditioned.
- The content of a pack, which was opened by mistake, should be considered as non-sterile. It should be made clearly visible that a package was opened. E.g. by clearly damaging the packaging material. The content of such pack shall be inspected and when necessary, cleaned. It then is repacked and sterilized.
| TOP | CONTENTS | PREVIOUS | NEXT | BOTTOM |
Packaging materials
A wide range of materials used for packaging of sterile supplies are available.
Traditionally, packaging materials for sterile supplies were reusable, such as sterilizing drums and cotton ware. Due to their inadequate microbial barrier, most of these traditional materials do not meet the requirements for primary sterile packaging anymore. They may still play a role as mechanical protection or additional dust protection layer. At the moment non-wovens, laminated film pouches, paper bags and containers are used as primary packaging materials. The following is an overview of packaging materials in use in sterile supply:
Soft/flexible packaging materials |
|
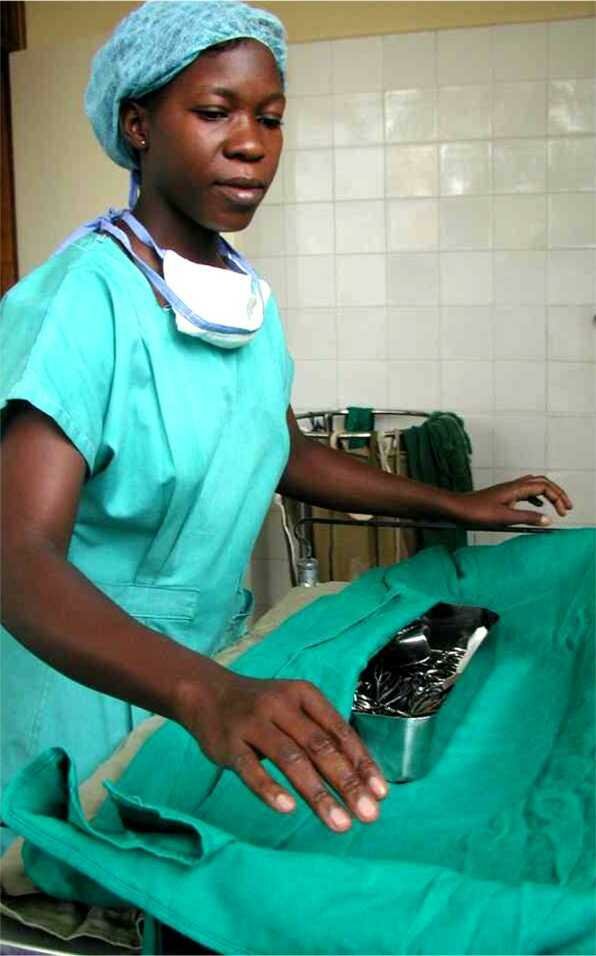 Packaging in cotton still being used in health facilities with limited resources |
Textile sheets: cotton or linenUse: Inner wrapping of instrument sets or outer dust protection Textile alone is not suitable as primary packaging! Cotton sheets have long been the standard packaging material for sterile goods. It has some major advantages
The openings between the threads however are larger then most micro-organisms and thus the fabric does not provide an adequate microbial barrier. It therefore does not meet the requirements anymore as primary packaging for sterile goods. They however are still often used as an inner wrapping for protection or as an outer dust cover. Whenever textile is used, it should contain its natural humidity. (It should be conditioned). If textile is too dry it may cause overheating of the steam and thus cause a failing sterilization. |
 Paper sheets available in many sizes and strengths |
Paper sheetsUse: Primary packaging for wrapping of textile packs and instrument sets in trays. Also used as inner packaging in containers Paper was the first alternative that replaced textile. It has a smaller pore size then textile. And thus can be used as primary packaging. Smooth papers are used for inner packaging, whereas crepe paper is stronger and is rough. Crepe paper can be used for inner and outer packaging. During sterilization, steam penetrates through the packaging. When paper is wet, it looses much of its original strength. Therefore stress in paper should be prevented. Wrapping should not be too tight, but also not too loose. It is essential that drying is adequate. Paper sheets are for single use only. |
 Paper sterilization bags |
Paper sterilization bagsUse: For packaging of individual instruments or small sets used in nursing stations and wards. Closing is usually done in a sealing device. Disadvantages:
Aseptic presentation can be improved by putting instruments in the bag with the handle at the opening end. It is not convenient to take out instruments from the bag. Its use has decreased with the introduction of laminated film pouches. Paper sterilization bags are for single use only |
 Non-woven wrapper  Non-wovens are water-repellent  Packaging an instrument set in a non-woven sheet using the parcel fold |
Non-woven sheetsUse: Primary packaging for wrapping of textile packs and instrument sets in trays. Also used as inner packaging in containers Non-wovens contain a certain amount of synthetic fibers. Added may be inorganic, textile, cellulose or other kind of synthetic fibers. These fibers of different materials are joined together, by for example pressing and heating. This means that the fibers are not woven together, but sealed together. For sterilization special non-wovens have been developed to meet the requirements for the primary packaging of sterile goods. They combine the good characteristics of other packaging materials:
Non-woven sheets are for single-use only |
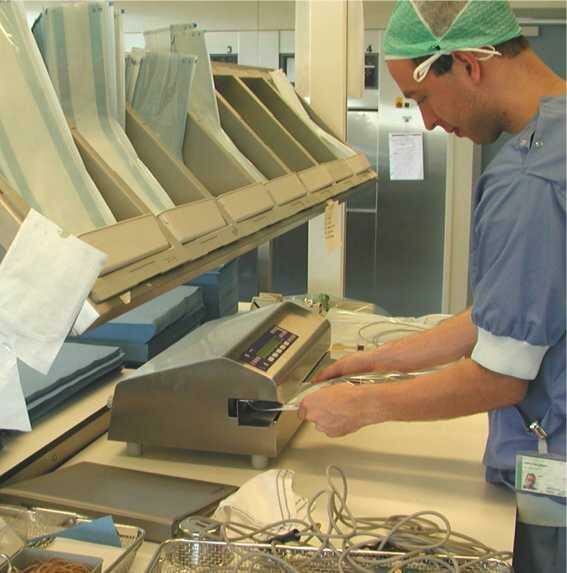
 Typical laminated film pouch  Laminated film pouches in various sizes and on roll  Sealing device for pouches |
Laminated film pouchesUse: Primary packaging for individual instruments or small instrument sets. They were the follow up of paper sterilization bags. The pouches consist of a sheet of paper or non-woven and a sheet of laminated transparent plastic, which are sealed together. The film cannot be penetrated by steam or air. Removal of air and penetration of steam is through the paper/non-woven. The pouch is opened by peeling back the laminated sheet from the paper sheets, like opening a banana. Pouches are available in many sizes. The open end of the pouch is closed with a sealing device. It is essential that the sealing temperature and pressure are adjusted well in order to get an adequate seal. Also laminated film packaging is available on roll. The user can cut pouches to any size needed. In that case both sides need to be sealed by the user. Remarks
|
Rigid packaging systems |
|
 Sterilizing drums are only suitable as additional mechanical protection (secondary packaging) |
Sterilizing drumsUse: As secondary packaging layer: mechanical protection of its content Not suitable as primary packaging. In the past, most sterilizers had vertical, round chambers. For various chamber sizes, drums were available. To allow steam into the drum, the wall is perforated. A metal band around the drum can be shifted in order to open and close the perforations. Before sterilization they are opened. After sterilization the perforations are closed again. They are known as Schimmelbush drums and were widespread. Problems:
Therefore they are not suitable as primary packaging. They may only be used as an extra mechanical protection. Drums with filters in top and bottomDrums are available with filters in the lid and bottom. They can be considered as sterilizing containers for round, vertical autoclaves. |
 Loading a sterilizer with containers  Sterilizing container 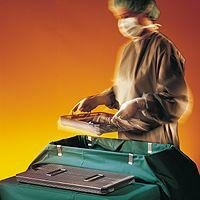 Taking out a tray from container |
Sterilizing containersUse: Primary packaging of textile packs and instrument sets in trays. Well designed sterilizing containers offer the following features:
Depending on the sterilizer process, containers shall be used with filters in top and bottom (for downward displacement process) or containers with filters only in the top (fractionated prevacuum process). |
| TOP | CONTENTS | PREVIOUS | NEXT | BOTTOM |
Wrapping techniques for packaging using sheets
When opening a pack containing sterile materials, it is essential that, due to the act of opening, the content is not contaminated. Wrapping techniques for packs and sets have been developed which assures this aseptic opening. The most common wrapping techniques that are applied for packaging of textile packs and instrument sets are the envelope fold and the parcel fold. The unfolded wrapper covers the instrument table and thus provides a sterile field. The techniques can be used for sheets of textile, paper and non-wovens.
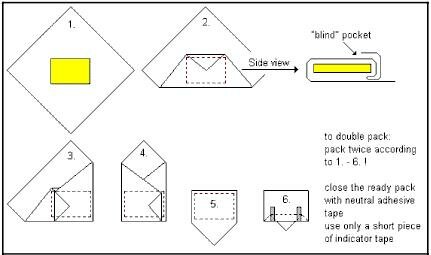
Envelope fold: For smaller objects and instrument sets.
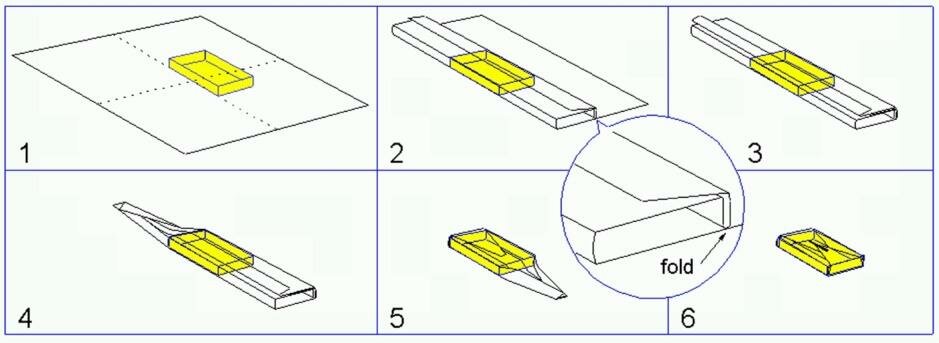
Parcel fold: Used for bigger packages such as instrument trays, textile packs etc.
| TOP | CONTENTS | PREVIOUS | NEXT | BOTTOM |
Accessories for packaging
Indicator tape
 Tapes for packaging suitable several agents. With and without indicator |
To keep a package together it is taped with an adhesive tape. This may be tape with an indicator or without indicator. A tape should have good adhesive qualities, should be easy to remove, and should not leave any residues If the tape has an indicator, it should show a clear colour change when exposed to a sterilization process. The tape with the indicator is known as indicator tape. When used for steam it is also referred to as autoclave tape. |
Trays and baskets
 Instrument set in tray |
For arranging sets of instruments, stainless steel perforated or mesh trays can be used. They are available in a number of sizes. In many cases, their size is based on the Sterilization Unit (30cmx30cmx60cm). This ensures that they fit efficiently in sterilizers, washer/disinfectors, and match sizes of packaging materials. |
 Basket as protection for small packages |
Baskets can be used for smaller packs and provide extra protection for the primary packaging during handling. Moreover they keep smaller packs upright, in order to allow optimal access of the sterilizing agent to the packs. Also the sizes of baskets usually are based on the Sterilization Unit making them compatible with tray sizes, sterilizers and washer-disinfectors. |
Protection materials
 Instrument protection |
Protection materials can be used to prevent that sharp instruments cause damage to the primary packaging. Also the instrument itself is protected against damage They should not be too tight around the instrument, so that the sterilizing agent can reach the surfaces of the instrument |
Dust covers
 Pouch in dust cover |
Dustcovers are applied around the primary package to providing extra protection and may extend the expiry period. The dustcover should be applied after the load has cooled down sufficiently, earliest 30 minutes after sterilization! |
Workstation for packaging
 Workstation for packaging in pouches |
Packaging is a job, which requires careful attention. Instruments, trays, and packaging materials need to be handled. That is why a packaging workstation should be designed in such a way that the work can be done efficiently and that physical problems of the workers are prevented. A table of a suitable size and height, with easy access to all required materials is indispensable. |
| TOP | CONTENTS | PREVIOUS | NEXT | BOTTOM |
Choice of materials and methods for packaging of sterile supplies (primary packaging)
Depending on what is to be packaged, a choice is made for the materials and method of packaging. Several countries have different opinions. In many cases the manufacturer of instruments and other medical supplies may give specific instructions for packaging and sterilization. In such case these instructions should be adhered to. Any combination of load, its packaging and sterilization process should be validated. The following can serve as a guideline for steam sterilization:
Textile packs
Recommended: Two sheets of packaging materials; parcel-fold or envelope fold
Alternative: Container, laminated film pouch
Small quantities of textile and/or bandages/swabs
Recommended: Laminated film pouch, possibly in dual laminated film pouches
Alternative: container
Instrument sets in trays/baskets
Recommended: Two sheets of packaging materials: parcel fold or envelope fold
Alternative: Container, laminated film pouch
Individual instruments:
Recommended: Laminated pouch, possibly in dual laminated film pouches
Alternative: container
Bowls and trays (small)
Recommended: laminated film pouch; paper sheet, paper bag
Alternative: container
Bowls and trays (large)
Recommended: Two sheets of packaging materials: parcel fold or envelope fold
Alternative: laminated film pouch, paper bag, container
Catheters, tubing, hoses
Recommended: Laminated film pouch, if appropriate in dual laminated film pouches
Alternative: two sheets of packaging material, paper bag, container
Scopes
Recommended: Special container, laminated film pouch, possibly in dual laminated pouches, laminated film pouch from roll.
Alternative: two sheets of packaging material, paper bag
Fine surgical instruments (individual as well as in sets)
Recommended: Dual laminated film pouches, special container, two sheets of packaging material in combination with a support system/rack
Also possible: paper bag
Mammal prosthesis
Recommended: sheet of packaging material in paper bag
Also possible: container, laminated film pouch
| TOP | CONTENTS | PREVIOUS | NEXT | BOTTOM |
Normative references (European)
Packaging materials should meet minimum requirements that have been formulated by the CEN (European Standards Commission):
|
EN 868-1 |
Packaging materials and systems for medical devices which are to be sterilized. General requirements and test methods |
|
EN 868-2 |
Packaging materials and systems for medical devices which are to be sterilized - Part 2: Sterilization wrap Requirements and test methods |
|
EN 868-3 |
Packaging materials and systems for medical devices which are to be sterilized - Part 3: Paper for use in the manufacture of paper bags (specified in Part 4 of this standard) and in the manufacture of pouches and reels (specified in Part 5 of this standard) - Requirements and test methods |
|
EN 868-4 |
Packaging materials and systems for medical devices which are to be sterilized - Part 4: Paper bags - Requirements and test methods. |
|
EN 868-5 |
Packaging materials and systems for medical devices which are to be sterilized - Part 5: Heat sealable pouches and reels of material manufactured from paper and plastic Requirements and test methods, |
|
EN 868-6 |
Packaging materials and systems for medical devices which are to be sterilized - Part 6: Paper for the manufacture of packs for medical use for sterilization by ethylene oxide or irradiation - Requirements and test methods. |
|
EN 868-7 |
Packaging materials and systems for medical devices which are to be sterilized - Part 7: Adhesive coated paper for the manufacture of heat sealable packs for medical use for sterilization by ethylene oxide or irradiation - Requirements and test methods. |
|
EN 868-8 |
Packaging materials and systems for medical devices which are to be sterilized - Part 8: Re-usable containers for steam sterilizers conforming to EN 285 - Requirements and test methods |
| TOP | CONTENTS | PREVIOUS | NEXT | BOTTOM |
© 2006 WFHSS