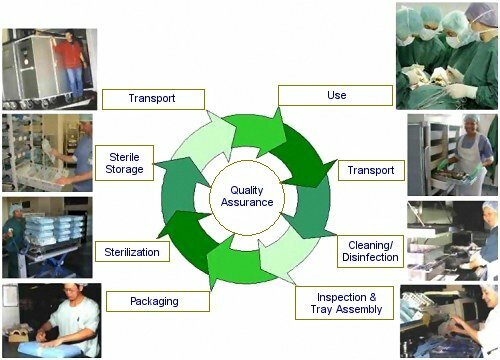
The Sterile Supply Cycle
Author: Jan Huys
 |
Importance of sterilization of medical supplies
More and more, infectious diseases form a serious threat to peoples health. Adequate sterile supply plays an essential role in the attempt to reduce the spreading of diseases within the health service. In a number of short articles, each step in the cycle of sterile supply as it is performed in most health facilities is explained. This first article describes an overview of the sterile supply cycle. In the year to come, further descriptions of each step are worked out and will be presented by clicking on the topic title or the image of the respective step in the illustration above. Detailed information on each topic can be found in more extensive professional literature and the international standards related to sterilization.
Introduction: The cycle of sterile supply
People come to health facilities to be cured from disease and injuries. Many of their diseases are caused by micro-organisms. Therefore health facilities are places with a high incidence of disease-causing micro-organisms which are easily spread from patient to patient by the staff and equipment and other materials used for patient care. Moreover many people visiting hospitals are weak and therefore are extra susceptible for acquiring a disease. It is the task of the health facilities not only to cure diseases of its patients but also to prevent transmission of diseases from one patient to the other. An important measure against spreading of diseases is the requirement that all medical supplies, such as instruments, swabs, drapes etc, which are used on open wounds or will be in touch with the inner fluids of the body, are free of any viable micro-organisms. They have to be sterile. Some of these materials are sterilized at the factory and are designed for single use. However, many instruments and materials used for medical interventions are very expensive and are designed such that they can be re-used. A high-quality reprocessing cycle is necessary in which the used materials are treated such, that they can be used safely again. As the reprocessing of sterile goods has developed into a specialism on its own, reprocessing should centralized in a single Central Sterile Supply Department (CSSD) serving the whole facility. In mostly older health facilities sterilization is still decentralized. More and more the sterilization activities of these departments are put under the responsibility of a single sterilization professional in charge of sterile supply of the whole facility.
|
|
|
 |
Transportation to the sterilization departmentAfter use, for example in the operating theatre or other treatment room, the soiled materials are collected and transported in suitable containers and trolleys to the location where the reprocessing takes place: the Central Sterile Supply Department. |
|
|
|
 |
CleaningThe instruments and materials are taken to the cleaning section of the sterilization department. In the cleaning section the dirty materials are handled; therefore this area is know as the dirty area of the sterilization department. Cleaning implies the removal all (visible) debris and dirt. The large majority of micro-organisms including any disease-causing agents are removed here. Adequate cleaning is considered the most essential step in the reprocessing cycle of sterile goods. [ FULL ARTICLE ] |
|
|
|
 |
Inspection and tray assemblyA missing or failing instrument while performing a surgical procedure is the annoyance of any surgeon! It can be the cause of great problems, for the patient as well as the staff performing an operation. It is therefore essential that instrument trays for all procedures are complete and that each instrument works correctly. That is why each individual instrument is subjected to a vigorous inspection, and that each tray should be double-checked for completeness. |
|
|
|
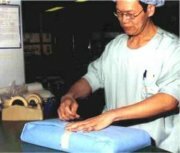 |
PackagingBefore they are used, sterile goods are usually stored until they are needed. In order to prevent recontamination during storage, they have to be packed. This also implies that the load is to be sterilized inside its packaging. Therefore the packaging should allow for the sterilizing agent to reach the actual load. Whereas after sterilization, it should prevent that micro-organisms can reach the items inside; it should act as a microbial barrier. Packaging should guarantee sterility upto the moment a product is used. Poor or damaged packaging makes the all the work of cleaning, packaging and sterilization useless! [ FULL ARTICLE ] |
|
|
|
 |
SterilizationAfter packaging the load is ready to be sterilized. In a sterilizer the micro-organisms remaining after the cleaning process are killed. Their number is reduced to a probability, which is considered safe: the Sterility Assurance Level. A range of methods are in use, all with their specific field of application: moist heat, dry heat, ethylene oxide, formaldehyde, irradiation, and gasplasma. The most common and safe method used in health facilities is the sterilization by moist heat using pressurized high-temperature steam. The machines used for sterilization with steam are known as steam sterilizers or autoclaves. Sterilizers should meet the stringent technical standards for performance and safety (for example the European norm EN 285 for Large Steam Sterilizers). To ensure the safety of the staff and patients, for each sterilizer used for medical supplies, all processes in combination with each type of load in its packaging should be validated. Simply said: You have to prove that your sterilizer sterilizes. |
|
|
|
 |
Sterile StorageUpon completion of the sterilization cycle, the goods are taken out of the sterilizer. Based on the registered process data and indicators the cycle is checked and when the required conditions are met, the load is released for storage, transport and use. The sterile goods are stored in a dedicated storage area, where they are kept until they are taken away for the next use. In a sterile storage there are special requirements for environmental conditions and stock management. A regime of product shelf life or the concept of event related sterility is used to ensure the integrity of each sterile item until its use. |
|
|
|
 |
Transportation to the userWhen sterile goods are needed they can be requested to be picked up from the sterile storage and are transported in dedicated closed trolleys or container systems to the locations where they are needed. When materials are to be transported outside the facility, additional measures are to be taken to ensure the integrity of the materials and an adequate protocol is necessary for handing over of the goods to the end-user. |
|
|
|
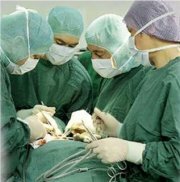 |
Use of sterile productsAny sterile product needs to be used correctly to ensure its safe use on a patient. By simply opening the sterile package wrongly, the instruments can be contaminated, just before they are used& By considering the concept of aseptic procedures, the chances on recontamination at the moment of using sterile goods is to be reduced to a minimum. The aseptic opening of a sterile pack and the presentation of an instrument to the surgeon are examples of such procedures. |
|
|
|
  |
Quality AssuranceEach step in the sterile supply cycle is crucial to a good and safe use of a sterile instrument or other item during a medical intervention. A mistake or failure in any of the steps may cause recontamination and makes the whole procedure useless. It may result in huge costs and can cause serious suffering and even endanger the life of patients and staff. That is why each step shall be subjected to vigorous monitoring. This is realized through a Quality Assurance system, in which each step in the cycle is analyzed, documented and monitored. It thus is a tool, to deliver a product that meets predefined quality standards which implies the provision of sterile supplies that are safe to use for patients and staff and perform the function they are intended for against an acceptable price. |
© 2006 WFHSS " Updated: 13 June 2006, 10:30 [GMT]